Mammalian Toxicology, Session 3
Organismal regulation,
feedback circuits; C 20, 21; Cell cycle controls: current models; C 3
General
Comments:
While Casarett &
Doull is a very good text for toxicology, there
are some areas, particularly of basic physiology that are not explained
as well as I might hope them to be. Endocrine and neural
communication
are two of these areas of minimal coverage in C& D that govern much
of organismal regulation. I strongly suggest that you review some
of
these
materials in any physiology, biology, biochemistry, cell biology,
neurobiology
and/or endocrinology texts that you may have from previous course-work
or reading. Many illustrations, notes, and links pertinent to
endocrinology
can also be found at http://kcampbell.bio.umb.edu
which is a public access site available on a 24/7 basis via one of the
central University of Massachusetts at Boston servers. (Many of
the
illustrations
below come from that site and may be accessed via the following links.)
Campbell
Reproductive Biology Site
Endocrinology
Lecture Illustrations
This page contains the
illustrations that were or are going to be
used
in Dr. Campbell's Endocrinology
Lecture
Course. These illustrations, etc. may still be useful to
others
as they apply to many subjects used in biology.
Return to Site
Directory
or Endocrinology
Lecture.
- What is
Endocrinology
- Endocrine
Functions
- Components of
Communication
Systems
- Endocrine
Analogs of
Communication Components
- Known
Hormonal Classes
- Definition of
a Hormone
- Signal
System Types
- Hormonal
Sources
- Hormonal
Sources II
- Brain
Anatomy
- Limbic
System Association
- Hypothalamic
Nuclei Function
- Hypothalamus
&
Posterior Pituitary Associations
- Neurophypophysis
Circulation
- Adenohypophysis
Circulation
- Pituitary
& Hypothalamic Anatomy
- Gross
Pituitary Histology
- Gross
Anatomy
of the Adrenal
- Microanatomy
of the Adrenal Cortex
- Information
Content
and Signal Fluctuation
- Signal
Pulsatility
- Controls on
Bioavailable
Hormone Levels
- Hierarchical
Systems
of Control
- Postive and
Negative
Control Loops
- Receptor
Types
- Properties
of Receptors
- Receptor
Notes
- Transduction
System
Properties
- Transduction
Notes
- Notes
on
Transduction Systems
- Hormone and
Receptor
Evolution
- Hormone-Receptor
Promiscuity
- Insulin
Family
Structures
- Insulin
Molecular Structure
- Evolution
of Insulin
Family Hormones and Receptors
- Relative
Affinities
in the Insulin H-R Group
- Assessment
of
Endocrine Function
- Serpentine
Receptors
- Cytokine/Growth
Factor Receptors
- Acetyl
Choline
Receptor
- Intracellular
Receptors
- Nuclear
Receptor
Response Elements
- Binding
of
Nuclear Receptors to DNA
- Bioassay
Dose-Response
- Biphasic
Dose Response of GH
- Bioassay
Notes
- Chemical
Assay
Notes
- Assay
Parameters
- Antibody
Binding to
Epitopes from Davidson College, MA Campbell
- Antibody
Assay Notes
- Competititve
Immunoassay Characteristics
- Competitive
Immunoassay Error Distributions
- Competititve
Immunoassay Estimation Errors
- Immunoassay
Precision Profile
- Competitive
Immunoassay Precision Profile
- Competitive
Immunoassay, Parallelism
- Noncompetitive
Immunoassay Characteristics
- Cell
and
Receptor Sizes
- Adjusting
Cellular Response Sensitivity
- Receptor
Binding and Numbers of Receptors
- Impact
of Losing Receptors on Biological Response
- Hypothalamic
Sources of Releasing Hormones
- Adenohypophysial
Hormones and Regulators
- Protein
Hormone Production
- Images for
Review of
Cell Physiology and Biochemistry
- Endoplasmic
Reticulum Role in Protein Synthesis
- Golgi
Actions in Protein Synthesis
- LH,
FSH,
TSH, hCG Introduction
- More
on Glycoprotein
Hormone Comparisons
- Yet
More
on Glycoproteins
- LH
Bioassay
Setup
- Proopiomelanocortin
Metabolism
- TSH
Control
- ACTH
Control
- Adrenal
Function
- Adrenal
Function & Regulation
- MSH
Control
- FSH
Control
- LH
Control
- GH
Control
- PRL
Control
- GH and
PRL Gene
Properties
- Pituitary
Testituclar Axis
- Spelling
Is Important!
- Large
G Proteins
and Protein Kinase A Cascade
- A
cAMP Cartoon
- Guanylyl
Cyclase Activation
- Large
G Proteins
and Protein Kinase C Cascade
- Glyceride
Chemistry
- Phosphoinositide
Metabolism
- Small
G Proteins
and Tyrosine Kinase Cascades
- Growth
Factor/
Tyrosine Kinase Pathway (Examples)
- Transduction
Mechanism Networks
- Insulin
and
Related Receptor Mechanisms
- Oncogenesis
Notes
- Cell
Cycle Control
Points
- Restriction
Point Switch
- Cancer
Genes
- DNA
Replication
- DNA
Replication
Fork
- Lipoprotein
Metabolism
- Receptor
Mediated
Endocytosis
- Steroid
Structure
- Steroid
Synthesis
- Steroid
Hormones of the Reproductive System
- C21
Metabolic Pathways
- C19 &
C18 Metabolic
Pathways
- Cellular
Steroidogenesis
- STAR
Protein
- Enterohepatic
Circulation
- Introduction
for
Reproduction
- Images
from Veterinary Reproductive Endocrinology
- Cell
Division Notes
- Meiosis
- Prophase
Meiosis I
- Meiosis
I
and II beyond Prophase I
- Gametogenesis
Outline
- Male
Reproductive
Anatomy
- Testis
Anatomy
- Seminiferous
Tubule
Gross Histology
- Seminiferous
Tubule
Microanatomy
- Seminiferous
Tubule
Closeup
- Seminiferous
Tubule SEM
- Seminiferous
Tubule Architecture
- ABP Notes
- Stages
of Spermatogenesis
- Spermatogenesis
- Sperm
Cytology
- Epididymal
Sperm Notes
- Capacitation
and Acrosome Reaction Notes
- Female
Reproductive
Anatomy
- Menstrual
Cycle
- Fertile
Phase
- Oogenesis
- Ovarian
Germ Cell Numbers
- Female
Gamete
Development
- Folliculogenesis
- Primordial
Follicle
Histology
- Primary
Follicle
Histology
- Secondary
Follicle Histology
- Graafian
Follicle
Histology
- Follicle
Dynamics
- Follicular
Estrogen Synthesis: 2 Cell Model
- Corpus Luteum
Histology
- Proliferative
Phase Uterine Histology
- Secretory
Phase
Uterine Histology
- Vaginal
Epithelial
Histology
- Gamete
and
Zygote Transport in the Oviduct
- Fertilization
Site
- Fertilization
- Fertilization:
An Illustrated Outline
- Sperm-Egg
Fusion
- Initial
Stages of Zygote Division & Development
- Luteal
Lifespan
& Luteolysis: Nonfertile Cycle
- Counter-Current
Delivery of Prostaglandins to the Ovary from the Endometrium
- Maternal
Recognition of Pregnancy; Luteal Lifespan: Fertile Cycle
- Nidation,
Early Stages
- Nidation,
Late Stages
- Normal
Profiles
of Hormones of Pregnancy
- Steroidogenesis
by the Maternal-Feto-Placental Unit
- Embryology
& Organogenesis in the Primate
- Sex
Determination
in Mammals is a Process
- SRY
Is the
Sex Determining Gene in Mammals
- Molecular
Biological Cascade Involved in Gonadal Formation
- Gonadal
Differentiation
- Differentiation
of the Internal Reproductive Phenotype
- Development
of the External Reproductive Phenotype
- Term
Placenta
Villi Histology
- Prostaglandin
Metabolism & Childbirth Initiation
- Pregnancy
& Childbirth
- Parturition
- Descriptive
Anatomy of the Breast
- Hormonal
Control of Breast Development
- Cellular
Organization of the Breast Alveolus
- Progesterone
Inhibition of Milk Production in Pregnancy
- Nonlactating
Breast Histology
- Lactating
Breast
Histology
- Initiation
of Puberty & LH Changes during Adolescence
- GONADOSTAT
Theory of Pubertal Onset
- Normal Thyroid
&
Goiter Anatomy
- Schematic
of Thyroid Cellular Anatomy
- Biosynthesis
of Thyroid Hormones by the Thyroid Follicular Epithelial Cells
- Chemistry
of Thyroid Hormone Biosynthesis
- Thyroid
Hormone Mechanism of Action
- Schematic
of Gross Pancreatic Anatomy
- Pancreatic
Histology
- Schematic
of Pancreatic Islet
- Islets of
Langerhans
Histology
- Hormones
from the Pancreatic Islets
- Notes
on
Pancreatic Hormones
- Simplified
Schematic of Glucose Homeostasis
- Hormonal
Impacts on Glucose Homeostasis
- Some
Introductory
Notes on Diabetes
- Satiety
- Homeostasis
of Blood Pressure Control, Water & Sodium Balance
- The
Juxtaglomerular
Apparatus & Renin Production
- Metabolism
of Angiotensinogen & Angiotensin
- The
Physiological
Problem of Glucocorticoid Binding to Mineralocorticoid Receptors
- Kallikrein
Metabolism
- Integration
of Kinin and Renin Metabolism
- Effectors
of Aldosterone Action
- Calcium
Homeostasis
- Metabolism
of Cholecalciferol
- Bone
Cellular Anatomy, Sagittal View
- Bone
Cellular Anatomy, Cross Section
- Calcium
Movements Associated with
Osteoid Cells
With respect to
original journal article sources that might be used in
the course or as material for your projects, the following list of
journals
may be helpful. Most of these can be found at the libraries of
the
Boston
Consortium institutions (UMB, Tufts, Boston-College, Boston University,
MIT,...) via library catalog searches. They are also available
via many
medical schools and schools of public health including
Umass/Worcester.
If you have Uncover access via school or work, you will be able to
order
specific articles you might find via PubMed or some other literature
search
engine. If there are items you are having difficulty finding or
obtaining,
please contact me and we will arrange to get them via services to which
I have access. Martingale's is also a very useful site both for
toxicology
information and just about any other type of scientific literature or
information
you can imagine.
Adverse
Drug
Reactions and Toxicological Reviews
Annual Review of
Pharmacology
and Toxicology
Applied
Occupational and Environmental Hygiene
Archives
of Environmental Contamination and Toxicology
Archives
of Toxicology
Bulletin
of Environmental Contamination and Toxicology
Cell
Biology
and Toxicology
Chemical
Research
in Toxicology
Chemico-Biological
Interactions
Chemotherapy
Comments
on Toxicology
Critical
Reviews in Toxicology
Current
Advances in Toxicology
Drug
and Chemical Toxicology
Ecotoxicology and
Environmental Safety
Environmental Toxicology and
Chemistry
Environmental
Toxicology and Pharmacology
European Journal of
Genetic Toxicology
Experimental
and Toxicologic Pathology
Food
Additives and Contaminants
Fundamental and
Applied
Toxicology
Human and
Experimental
Toxicology
Immunopharmacology
and Immunotoxicology
In Vitro &
Molecular Toxicology
Inhalation
Toxicology
International
Journal of Toxicology
Journal of Analytical Toxicology
Journal
of Applied Toxicology
Journal
of Biochemical and Molecular Toxicology
Journal of
Environmental
Pathology, Toxicology and Oncology
Journal
of Toxicology - Cutaneous and Ocular Toxicology
Journal
of Toxicology and Environmental Health Part A
Journal
of Zoological Systematics and Evolutionary Research
Neurotoxicity
Research
Pharmacology
Pharmacology
& Toxicology
Regulatory
Toxicology and Pharmacology
Reviews in
Toxicology
Toxicologic
Pathology
Toxicological
& Environmental Chemistry
Toxicological Sciences
Toxicology
Toxicology
& Environmental Health Part A
Toxicology and
Applied
Pharmacology
Toxicology
and Industrial Health
Toxicology
Arena
Toxicology
in Vitro
Toxicology
Letters
Toxicology
Methods
Toxicology Modeling
Toxicon
Organismal
Regulation:
Much of organismal
regulation is concerned with maintaining
homeostasis,
both internally and with respect to responses to external
stimuli. Both
the central nervous system (CNS) and the endocrine system play the key
roles in this network of communication. The CNS uses many of the
same
chemical
signaling mechanisms that the endocrine system does, but tends to
employ
them in the more localized environment of the synaptic cleft or the
neuromuscular
junction. In addition the nerves of the CNS employ electrical
depolarization
to an extent much more clearly described in that tissue than in others
to cause cellular activation. While there is no doubt that this
electrochemical
network, as it functions in the nervous system and the tissues
activated
by it, plays a key role in controlling and regulating normal function
and
homeostasis and because of this is of great concern when evaluating the
actions of toxicants and toxins, I will limit most of my comments to
chemical
communications as they have been described in the endocrine
system.
That
this is not a terrible limitation is primarily due to the fact that
modern
endocrinology is really the study of chemical communications within
eukaryotic
organisms. As such it includes much of what happens in the CNS
and even
touches on much of what controls prokaryotic organisms.
First, a chemical
communication system consists of several parts all
of which are possible targets for toxic insult. A signalling cell
produces
a chemical signal (usually what we call a hormone
of
one sort or the other) which is secreted or shed into a nondestructive
carrier matrix (often plasma or intercellular fluid). Since the
job of
a hormone is only to convey information and since changes in needed
information
may change very rapidly, cells are conservative in the amount of energy
they invest in producing hormone molecules. As a result, they are
often
produced in very small amounts of the order of 10-9 to 10-15
M. These small unaltered chemical signals must be sensed by target
cells in the context of a complex chemical mixture that may
contain closely related molecules or metabolites. This is
accomplished
by a chemically specific receptor
that
usually resides either in the cell membrane or within the cell
nucleus.
The receptor is allosterically altered by binding of the hormone and
changes
its interactions with other proteins in the cell so as to cause a
sensation
of the hormone molecule. This is often done via specific
alterations in
transducer
proteins that associate with receptors and can directly generate
intracellular
secondary message chemicals or act on other protein and enzymatic
machinery
via allosteric interactions to produce an effector
protein response. Since the transducer proteins often have an enzymatic
activity, they amplify the original signal passed to them by the
receptor-hormone
complexes. This is further amplified by the effector proteins and
translated
into a change in cellular motion, shape, metabolism, gene
transcription,
or cell growth or division.
The chemical hormonal
signals come in a variety of molecular types,
but these can generally be classed as proteins
(e.g., prolactin -- PRL, insulin, thyroid stimulating
hormone/thyrotropin
-- TSH, growth hormone releasing hormone/somatoliberin -- GHRH), peptides
(e.g., glucagon, thyroid releasing hormone/thyroliberin -- TRH,
somatostatin
-- SS), amino acid derivatives
(e.g.,
neurotransmitters like epinephrine -- E/Epi, serotonin, dopamine -- DA;
thyronines/thyroid hormones like thyroxine -- T4, or
triiodothyronine
-- T3), lipids (e.g.,
steroids
like testosterone, estradiol, or progesterone, or eicosanoids like
prostaglandin
E2 or thromboxanes), or gases
(e.g., NO and CO). Evidence also indicates that there is a
specific
membrane-bound calcium ion receptor in some cells, thus making that ion
a candidate hormone. Proteins, peptides, neurotransmitters, and
charged
lipids like eicosanoids most often bind to receptors that are integral
membrane proteins that present a binding surface at the extracellular
face
of target cells. Thyroid hormones, steroids, and some
prostaglandins
all seem to bind primarily to intracellular receptors that are often
associated
with DNA in the cell nucleus. These hormones are lipophilic
enough
to diffuse through cell membranes. Likewise gases also diffuse
through
cell membranes after which they appear to act via binding and
allosteric
alteration of enzyme activities. Notice that the various chemical
properties of hormones tend to mimic the diversity of chemistries seen
in toxicant chemicals including synthetics. It is not surprising
then that these compounds can interact and cause alterations in many
physiological
systems since those systems evolved to handle a similar diversity of
useful
information signals.
Chemical signalling
also exists in a series of distinguishable
forms.
It may occur between two distant cell types via hormones secreted into
the blood, lymph, or intracellular fluid = endocrine
signalling.
It may occur between cells that are close to one another via the same
fluid
media = paracrine
signalling.
It may take place between adjacent cells (and may even involve signals
and receptors that are tethered to the surfaces of the interacting
cells)
= juxtacrine signalling. Or
it
may involve cells signalling to themselves or to nearby cells of the
same
type = autocrine signalling.
Note also that at
each level of chemical signalling modulation may
take
place in the normal course or development and functioning: hormone
levels
change over time; receptor levels change over development, time, and in
response to hormone levels; transducer and effector proteins
change
in level and activity with development, time, and hormone levels, they
may be phosphorylated or dephosphorylated, prenylated or deprenylated,
and associated with intracellular activators or inhibitors depending on
cellular status and condition. Moreover, although it is common
for
only a limited number of cells within a tissue or organism to have
receptors
for a particular hormone (chemical signal) it is also true that most
cells
in the body respond to several to many different hormones. A cell
may be responding to a steroid (via a nuclear receptor and altered gene
transcription) at the same time as it is responding to a
neurotransmitter
(via an ion channel coupled receptor) while it is also producing a
peptide
hormone in response to yet another protein hormone (via a cell membrane
spanning receptor). It is also true that the vast majority of
cells
in eukaryotes do make hormones or hormone-like chemical signals during
at least a portion of their lifespans. These systems are
incredibly
dynamic. So it is not surprising that toxicants can have so many
different possible routes to generate cellular disruption.
But because of this dynamism and interconnectedness, it is also quite
possible
to observe what appears to be a primary toxic insult in a tissue that
turns
out not to be the primary site of toxicant action. In fact, many
of the results covered in Chapters 20 and 21 of Casarett and Doull
could
almost be predicted based on our current knowledge of how chemical
communications
operate within the body.
A bit of description
about the various chemical communication
networks
may help illustrate this point.
Feedback
Circuits:
The target cell
response also frequently generates one or more
chemical
signals that now make this target cell into a signalling cell. The
secondary
hormonal signal may now impinge on other tissues to produce other
cell-specific
responses. Among these are frequently the original signalling
cells or
a set of cells that control those signalling cells. This
generates a
control
circuit that can now operate to balance hormonal outputs in proportion
to any other inputs into the cells of the control circuit as well as to
the original two hormones in the control circuit described. S uch
circuits
are of two types: negative feedback,
which is normally homeostatically balances internal chemistry and
cellular
functions such as gametogenesis; or postive
feedback,
which is usually associated with production of a physiological change
of
state, e.g., birth, milk let-down, ovulation. Similar
controls
also
occur within excitable cells where protein phosphorylation and
dephosphorylation
often serves as a negative feedback circuit for triggering and then
limiting
the action of a hormonal (or electrical) stimulus or where growth
factor
actions produce a spiral of effects leading to cellular division.
The classical
endocrine system includes several well-defined glands
such as the adrenal, the testes, the ovary, the thyroid, the thymus,
the
pituitary, or the pancreatic islets. Those peripheral tissues
that
are capable of producing steroids in substantial quantities, the ovary,
testis, and adrenal cortex, are under the control of specific cell
types
(gonadotropes, and corticotropes) in the anterior portion of the
pituitary
which is centrally located below the base of the brain and in a bony
pocket
(the sella turcica). The
pituitary
is connected to the base of the brain (the hypothalamus) via a
well-vascularized
stalk of tissue. Neurotransmitters, and several peptide and small
protein hormones are produced by various portions of the hypothalamus
and
are secreted into the blood that circulates to the anterior
pituitary.
These neuroendocrine hormones (e.g., DA, TRH, SS) bind to receptors on
the target cells in the anterior pituitary and stimulate or inhibit
their
production of protein hormone products. The pituitary protein
hormones
(follicle stimulating hormone -- FSH, luteinizing hormone -- LH, and
adrenocorticotropic
hormone/corticotropin -- ACTH) are secreted into the venous drainage of
the anterior pituitary and proceed to circulate to the peripheral
organs
of the body. When they bind to their target cells they stimulate
a variety of processes that increase production and secretion of
steroids
that are characteristic of the peripheral target tissue.
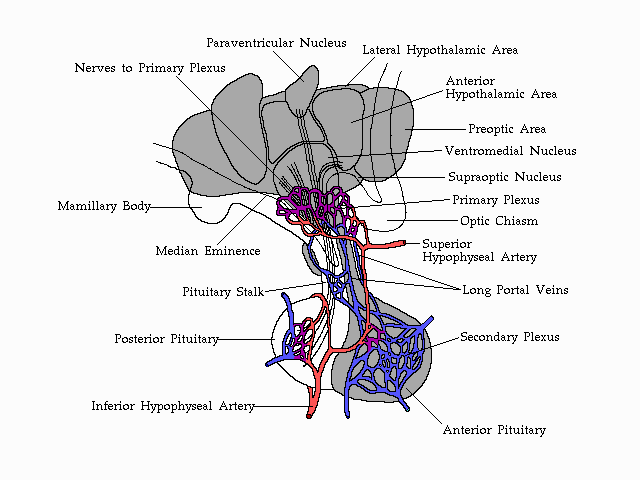 Vascularization
of the anterior pituitary and its association with the structures of
the
human hypothalamus.
Vascularization
of the anterior pituitary and its association with the structures of
the
human hypothalamus.
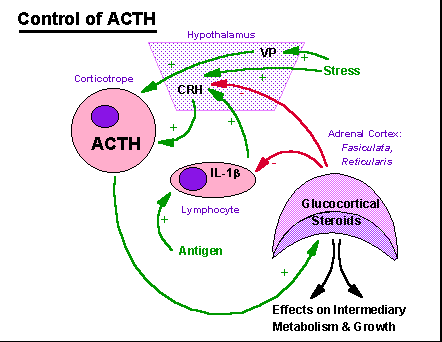 Control
of the
fasiculata and reticularis layers of the adrenal cortex by ACTH and of
ACTH by the glucocortical steroids produced by the adrenal cortex
(cortisol
-- human, corticosterone -- rat, mouse). CRH is corticotropin
releasing
hormone, a small protein produced in the hypothalamus. VP is
vasopressin,
a nonapeptide secreted by cells of the posterior pituitary and produced
by the cells of the paraventricular and supraoptic nuclei of the
hypothalamus.
IL-1B is a lymphokine, a protein hormone produced by lymphoid cells.
Control
of the
fasiculata and reticularis layers of the adrenal cortex by ACTH and of
ACTH by the glucocortical steroids produced by the adrenal cortex
(cortisol
-- human, corticosterone -- rat, mouse). CRH is corticotropin
releasing
hormone, a small protein produced in the hypothalamus. VP is
vasopressin,
a nonapeptide secreted by cells of the posterior pituitary and produced
by the cells of the paraventricular and supraoptic nuclei of the
hypothalamus.
IL-1B is a lymphokine, a protein hormone produced by lymphoid cells.
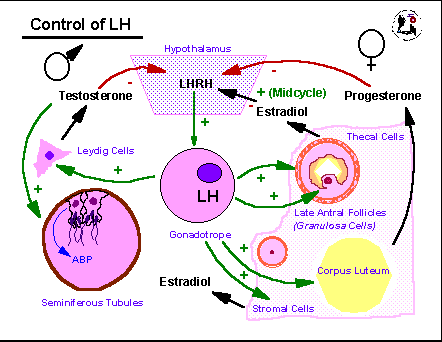 LH
acts in a simple
negative feedback loop in the male where it acts on the Leydig cells of
the testis to stimulate testosterone production. Testosterone
acts
on peripheral somatic tissues and is a major player in maintaining
adult
sperm production. The steroid also binds to receptors in the
basal
hypothalamus where it suppresses production of the decapeptide hormone
luteinizing hormone releasing hormone/luliberin, LHRH/GnRH. LHRH
in turn stimulates the anterior pituitary gonadotrope cells to produce
LH. In the female, progesterone from the temporary steroidogenic
structure formed from the ovarian follicle following ovulation, the
corpus
luteum, CL, acts in an analogous manner to testosterone. It
stimulates
peripheral tissues such as the uterine lining (endometrium) to
differentiate
in preparation for embryonic implantation but it also acts at the
hypothalamus
to decrease LHRH and subsequently LH production. Since the CL is
dependent on LH action for its function the negative feedback by
progesterone
will normally limit the lifespan of the CL and lead to its functional
and
structural involution. When this happens (in the absence of a
fertilization)
the decline in steroids associated with the CL involution leads to a
rise
in LH (and FSH). These initiate follicular growth in the adult
ovary
and stimulate production of estradiol from the granulosa cells of the
follicle
and the stromal cells of the ovarian tissue. As follicles grow
they
produce more and more estradiol which tends to act in a negative
feedback
manner initially similar to the actions of testosterone and
progesterone
with respect to LH and FSH production. Once a particular
threshold
is reached, however, in the later portion of the preovulatory part of
the
ovarian cycle, estradiol stimulates LH release by increasing the LHRH
receptor
numbers on the gonadotropes and, possibly, by inhibiting its own
negative
feedback action at the level of the hypothalamus. This positive
feedback
results in a spike of LH (and FSH) near the middle of the ovarian cycle
that triggers the shedding of ova from mature follicles and stimulates
conversion of the ovulated follicles into the next crop of CLs.
Glucocortical
steroids and CRH have suppressive actions on LHRH, LH, and sex steroid
productivity.
LH
acts in a simple
negative feedback loop in the male where it acts on the Leydig cells of
the testis to stimulate testosterone production. Testosterone
acts
on peripheral somatic tissues and is a major player in maintaining
adult
sperm production. The steroid also binds to receptors in the
basal
hypothalamus where it suppresses production of the decapeptide hormone
luteinizing hormone releasing hormone/luliberin, LHRH/GnRH. LHRH
in turn stimulates the anterior pituitary gonadotrope cells to produce
LH. In the female, progesterone from the temporary steroidogenic
structure formed from the ovarian follicle following ovulation, the
corpus
luteum, CL, acts in an analogous manner to testosterone. It
stimulates
peripheral tissues such as the uterine lining (endometrium) to
differentiate
in preparation for embryonic implantation but it also acts at the
hypothalamus
to decrease LHRH and subsequently LH production. Since the CL is
dependent on LH action for its function the negative feedback by
progesterone
will normally limit the lifespan of the CL and lead to its functional
and
structural involution. When this happens (in the absence of a
fertilization)
the decline in steroids associated with the CL involution leads to a
rise
in LH (and FSH). These initiate follicular growth in the adult
ovary
and stimulate production of estradiol from the granulosa cells of the
follicle
and the stromal cells of the ovarian tissue. As follicles grow
they
produce more and more estradiol which tends to act in a negative
feedback
manner initially similar to the actions of testosterone and
progesterone
with respect to LH and FSH production. Once a particular
threshold
is reached, however, in the later portion of the preovulatory part of
the
ovarian cycle, estradiol stimulates LH release by increasing the LHRH
receptor
numbers on the gonadotropes and, possibly, by inhibiting its own
negative
feedback action at the level of the hypothalamus. This positive
feedback
results in a spike of LH (and FSH) near the middle of the ovarian cycle
that triggers the shedding of ova from mature follicles and stimulates
conversion of the ovulated follicles into the next crop of CLs.
Glucocortical
steroids and CRH have suppressive actions on LHRH, LH, and sex steroid
productivity.
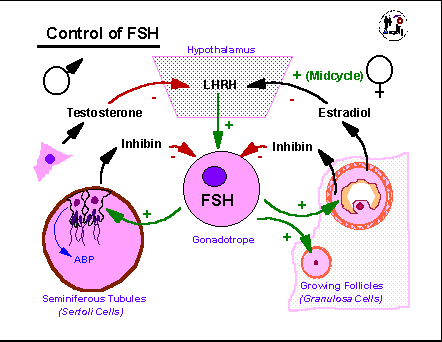 The
FSH control
cycle is intimately tied to that of LH and is only slightly less
complex.
In the male testosterone suppresses LHRH, LH, and FSH production while
being directly influenced only by LH. FSH stimulates the Sertoli
cells of the seminiferous tubules to produce along with the proteins
and
metabolic products needed to help support spermatogenesis, a protein
hormone,
inhibin, that acts in a direct feedback manner on the
gonadotropes.
Primary control of FSH is due to steroid feedback with only about 20%
of
levels critically dependent on inhibin. In the female, granulosa
cells produce inhibin in response to FSH as well as estradiol. So
growing follices are actually producing both a protein and a steroid
that
help suppress FSH production even as they depend on FSH actions to
continue
their growth and development. Again, primary control of FSH is
due
to steroid, rather than inhibin feedback. Once the estradiol
threshold
necessary to convert estradiol negative feedback into positive feedback
is reached, FSH levels also rise to a mid-cycle peak and then decline
as
progesterone takes over to suppress LHRH, LH, and FSH levels.
The
FSH control
cycle is intimately tied to that of LH and is only slightly less
complex.
In the male testosterone suppresses LHRH, LH, and FSH production while
being directly influenced only by LH. FSH stimulates the Sertoli
cells of the seminiferous tubules to produce along with the proteins
and
metabolic products needed to help support spermatogenesis, a protein
hormone,
inhibin, that acts in a direct feedback manner on the
gonadotropes.
Primary control of FSH is due to steroid feedback with only about 20%
of
levels critically dependent on inhibin. In the female, granulosa
cells produce inhibin in response to FSH as well as estradiol. So
growing follices are actually producing both a protein and a steroid
that
help suppress FSH production even as they depend on FSH actions to
continue
their growth and development. Again, primary control of FSH is
due
to steroid, rather than inhibin feedback. Once the estradiol
threshold
necessary to convert estradiol negative feedback into positive feedback
is reached, FSH levels also rise to a mid-cycle peak and then decline
as
progesterone takes over to suppress LHRH, LH, and FSH levels.
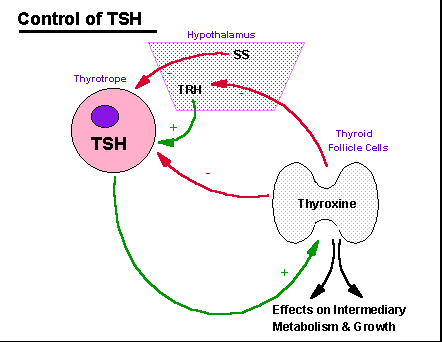 Control
of
thyroid function involves inhibition of the thyroptropes of the
anterior
pituitary by circulating thyroxine which acts directly as it does to
promote
growth and maintain metabolism on many somatic tissues in the
periphery.
Additionally, thyroxine can inhibit hypothalamically produced
TRH.
Somatostatin, a tetradecapeptide generated in the hypothalamus, can act
as a secondary controller by limiting the stimulatory actions of
TRH.
TSH from the thyrotrope then acts selectively on the follicular
epithelial
cells of the thyroid to stimulate the synthesis and release of
thryonines,
and especially thyroxine.
Control
of
thyroid function involves inhibition of the thyroptropes of the
anterior
pituitary by circulating thyroxine which acts directly as it does to
promote
growth and maintain metabolism on many somatic tissues in the
periphery.
Additionally, thyroxine can inhibit hypothalamically produced
TRH.
Somatostatin, a tetradecapeptide generated in the hypothalamus, can act
as a secondary controller by limiting the stimulatory actions of
TRH.
TSH from the thyrotrope then acts selectively on the follicular
epithelial
cells of the thyroid to stimulate the synthesis and release of
thryonines,
and especially thyroxine.
Controls for
production of growth hormone follow somewhat similar
patterns
to that for TSH and thyroxine, but prolactin is principally controlled
by the suppressive effect of hypothalamic dopamine. If production
of this neurotransmitter is limited in the neural circuits in the
hypothalamus,
PRL levels can rise in response to mammotrope/lactotrope (not
luteotrope)
production of the hormone. In the periphery this can affect
breast
tissue, immune, and reproductive tract functions. Centrally,
either
PRL or another controller of dopamine production, beta-endorphin, may
act
to affect other control circuits including LHRH production and,
thereby,
LH, FSH and gonadal function.
While control of
insulin and glucagon from pancreatic islets are
tied
to regulatory circuits involving the adipose tissue protein hormone
product
leptin, the gastrointestinal protein hormone GHrelin, and several
hypothalamic
hormones including CRH, control loops also occur that seem limited to
the
periphery. Control of calcium and phosphate metabolism by the
thyroid
hormone calcitonin and the parathyroid hormone, PTH, form one such
circuit.
Regulation of immune function often involves ACTH, CRH, and
glucocorticoids
so these complex circuits may be primarily peripheral, but do retain
important
ties to the hypothalamus and, thus, to the CNS.
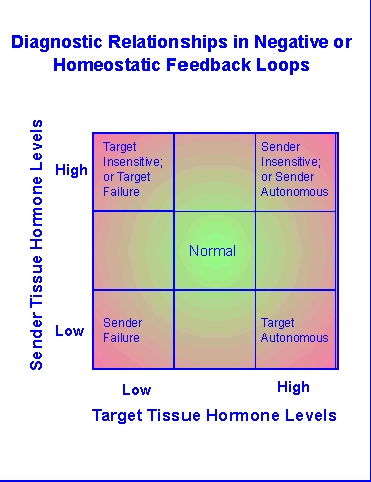 Disruption
of these circuits is a primary part of diagnostic investigation in
clinical
endocrinology. The methods used in such efforts have been and are
being used to explore toxicant insults in toxicological studies.
Diagramatically, problems that disrupt the usual negative feedback
circuits
of homeostatic endocrine tissues (e.g., thyroid, pancreas, adrenal
cortex)
will tend to cause one or more of the hormones involved in that circuit
to be too high (hyper-) or too low
(hypo-). The balance of
the
hormone
signals involved will often allow differentiation of where the circuit
is disrupted. It seems rather obvious that toxicants that impact
such circuits can also be investigated by evaluating hormonal endpoints
or using the techniques used in endocrine diagnostics.
Interestingly,
the focus on tumor formation as a key endpoint seems to have limited
the
use of the hormonal parameters and endocrine techniques to explaining
why
such tumors form rather than how such a disruption in the internal
homeostasis
within an organism might have disrupted normal function up to the time
of tumor formation and/or cancer production.
Disruption
of these circuits is a primary part of diagnostic investigation in
clinical
endocrinology. The methods used in such efforts have been and are
being used to explore toxicant insults in toxicological studies.
Diagramatically, problems that disrupt the usual negative feedback
circuits
of homeostatic endocrine tissues (e.g., thyroid, pancreas, adrenal
cortex)
will tend to cause one or more of the hormones involved in that circuit
to be too high (hyper-) or too low
(hypo-). The balance of
the
hormone
signals involved will often allow differentiation of where the circuit
is disrupted. It seems rather obvious that toxicants that impact
such circuits can also be investigated by evaluating hormonal endpoints
or using the techniques used in endocrine diagnostics.
Interestingly,
the focus on tumor formation as a key endpoint seems to have limited
the
use of the hormonal parameters and endocrine techniques to explaining
why
such tumors form rather than how such a disruption in the internal
homeostasis
within an organism might have disrupted normal function up to the time
of tumor formation and/or cancer production.
Cell
Cycle
Controls:
Current Models:
Although
altered
metabolism is a key element of homeostasis in any organism, the ability
to develop or repair tissues is dependent on the process of cell
growth,
mitotic division, and differentiation. And while we often think
of
this process as simply being regulated by availability of
nonavailability
of the nutrients necessary for cell division, the cellular machinery
involved
makes it essential that this process is highly regulated. Both
intracellularly
and extracellularly. This is not intuitive until several facts
concerning
DNA replication in eukaryotes are taken into account. First, DNA
replication starts from a common point on both of the complementary
strands
of the molecule and it proceeds from that point in both directions at
the
same time while using enzymes that synthesize DNA only in one direction
(5' to 3'). That involves construction of a continuous strand of
deoxyribonucleotides in one direction, but a discontinuous strand of
fragments
(first RNA, then DNA) in the opposite direction. These fragments
need to be ligated before the entire molecule is reconstructed.
Failure
to ligate them before the DNA fragments dissociate form their original
site and reassociate, possibly incorrectly, with a sequence of
similiar,
but not identical composition, can result in replicative
mistakes.
Second, eukaryotic DNA is not only helically coiled, but it is wrapped
around nucleosomal proteins, and further folded into chromosomal
structures
that retain substantial coiling even when they are not involved in cell
division. This coiling and supercoiling forces cells to cleave
their
DNA while they are replicating it in order to allow it to unwind far
enough
to provide access to the DNA polymerase/ligase complexes. Such
cleavage
can also lead to mistakes unless these sites are religated soon after
they
are cleaved. Third, the enzymes involved in DNA replication can
make
mistakes. This will occur most often if there is an uneven supply
of substrate nucleotides to the enzyme or if ionic composition modifies
the specificity of the enzyme, e.g., if Mg++ or Ca++
levels vary. In addition, these cells have evolved in an
environment
that contains potentially damaging agents, both chemical and
electromagnetic,
that can alter or cleave DNA during its time in the cell. As a
result,
cells have produced an array of enzymes that can remove damaged
segments
of one strand of DNA, replace them with new nucleotides, and ligate the
damaged segment back onto the parent strand. They can even
repair double strand breaks to a limited degree. Thus, monitoring
the condition of their own DNA and repairing it if needed is a normal
cellular
function. It is not, therefore, terribly surprising that similar
protein and enzymatic machinery is also used during cell replication to
evaluate DNA synthesis and condition prior to cell division. Nor
should it be surprising that failures in these functions tend to lead
to
problems in daughter cells leading most often to elimination of the
damaged
cell, but also occasionally to the production of cells that fail to
function
properly and undergo a transformation often accompanied by unrestrained
growth (neoplasia, tumorigenesis, carcinogenesis,
malignancy).
So how is mitotic cell division monitored or controlled?
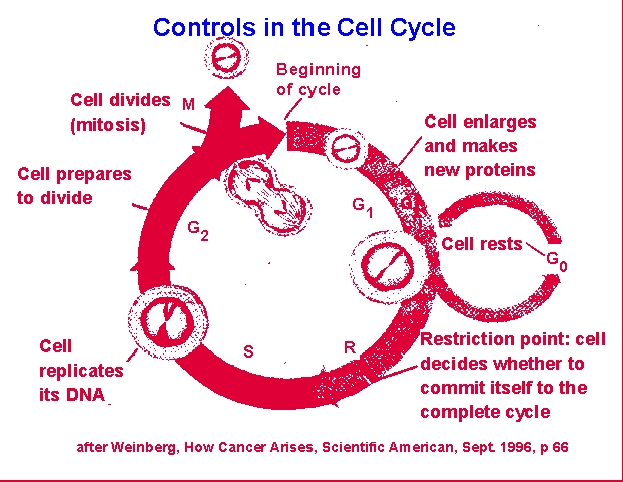 Cells tend
to be either in a resting or nonproliferative state, G0 or
they
are actively involved in some stage of mitosis. G1, or
gap 1, phase involves cellular growth and protein synthesis. S,
or
synthesis, phase involves protein and metabolite synthesis in
preparation
for cellular DNA synthesis and finally DNA synthesis itself. G2,
or gap 2, phase involves completion of DNA synthesis and reorganization
of the cellular constituents allowing for separation of chromosomes
(e.g.,
nuclear envelop breakdown, spindle organization). M, or mitosis,
phase involves the various segments of cell division: metaphase,
anaphase,
telophase, diakinesis. Note that prophase actually involves much
of the rest of the S and G2 phases. The "gap"
phases
refer to portions of the cycle during which tritiated thymidine does
not
incorporate into cellular DNA and cell structure cannot be used to
define
the cell's position in the cycle.
Cells tend
to be either in a resting or nonproliferative state, G0 or
they
are actively involved in some stage of mitosis. G1, or
gap 1, phase involves cellular growth and protein synthesis. S,
or
synthesis, phase involves protein and metabolite synthesis in
preparation
for cellular DNA synthesis and finally DNA synthesis itself. G2,
or gap 2, phase involves completion of DNA synthesis and reorganization
of the cellular constituents allowing for separation of chromosomes
(e.g.,
nuclear envelop breakdown, spindle organization). M, or mitosis,
phase involves the various segments of cell division: metaphase,
anaphase,
telophase, diakinesis. Note that prophase actually involves much
of the rest of the S and G2 phases. The "gap"
phases
refer to portions of the cycle during which tritiated thymidine does
not
incorporate into cellular DNA and cell structure cannot be used to
define
the cell's position in the cycle.
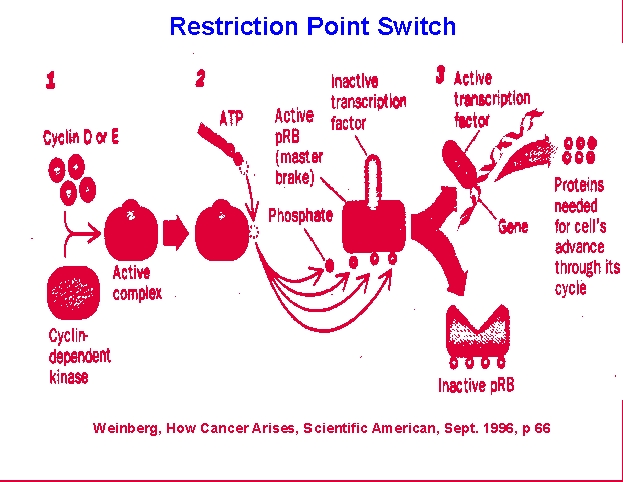 At the
point just before S phase there is the first of two checkpoints that
cells
use to monitor their condition and suitability to enter mitosis.
The restriction, or R, point also provides cells a means to allow
extracellular
input into the process of cell division. R involves the
retinoblastoma,
Rb, protein which acts as a brake on DNA synthesis unless growth factor
or other inputs allow an override of the brake. This can occur if
conditions in the organism stimulate growth factor production which
then
binds to cellular receptors and triggers phosphorylation of the Rb
protein
or if conditions within the cell provide adequate materials to allow
DNA
systhesis to be successful. The latter will allow accumulation of
cyclin/cyclin kinase complexes that can phosphorylate Rb. This
protein
then dissociates from the E2F transcription factor complexes needed to
promote DNA synthesis and allows DNA replication. The second
checkpoint
occurs just prior to commitment to mitosis, M, and involves the p53
protein.
This checkpoint allows the DNA repair proteins to complete any ligation
of damage or DNA-synthesis associated strand breaks. p53 binds to
complexes that permit movement beyond this point and is again subject
to
extracellular modulation by the products of growth factor
binding.
Once this suppressor protein is inactivated, mitosis can proceed and
daughter
cells can be produced.
At the
point just before S phase there is the first of two checkpoints that
cells
use to monitor their condition and suitability to enter mitosis.
The restriction, or R, point also provides cells a means to allow
extracellular
input into the process of cell division. R involves the
retinoblastoma,
Rb, protein which acts as a brake on DNA synthesis unless growth factor
or other inputs allow an override of the brake. This can occur if
conditions in the organism stimulate growth factor production which
then
binds to cellular receptors and triggers phosphorylation of the Rb
protein
or if conditions within the cell provide adequate materials to allow
DNA
systhesis to be successful. The latter will allow accumulation of
cyclin/cyclin kinase complexes that can phosphorylate Rb. This
protein
then dissociates from the E2F transcription factor complexes needed to
promote DNA synthesis and allows DNA replication. The second
checkpoint
occurs just prior to commitment to mitosis, M, and involves the p53
protein.
This checkpoint allows the DNA repair proteins to complete any ligation
of damage or DNA-synthesis associated strand breaks. p53 binds to
complexes that permit movement beyond this point and is again subject
to
extracellular modulation by the products of growth factor
binding.
Once this suppressor protein is inactivated, mitosis can proceed and
daughter
cells can be produced.
Should a cell fail
for some reason to heed these two checkpoints, it
could well pass partially broken DNA on to the daughter cells.
This
can lead to loss of important DNA segments or to introductions of
mistakes
when the DNA is repaired in the daughter cells. Since breaks
often
occur in areas associated with active gene transcription including
genes
for various portions of the signalling machinery for cell growth
factors
and their receptors, transducers, and effectors, mistakes may arise in
these important regulatory paths. If these result in constitutive
growth signalling, a cycle of inadequately regulated cell division and
gradually accumulating gene loss and transformation can occur as has
been
found to be the case in several forms of cancer in humans including
colon
cancer.
More frequently
problems in heeding the first checkpoint result in
shunting
of the cell toward programmed cell death or apoptosis.
This is often triggered by p53 activation or by the presence of
alternative
triggering paths like Fas/Fas ligand interactions. During toxic
insults
the path is often activated by the intracellular production of
oxidative
products such as peroxide or superoxide. Many indications seem to
point to the production of damage to the mitochondrial membrane and
intra-
as well as extracellular release of cytochrome C as a key element in
activating
transcription and translation of genes associated with the apoptotic
cascade
(e.g., caspases, Bax, Bcl) that leads to intracellular proteolysis,
nucleolysis,
organellar breakdown, and finally cellular dissruption by osmotic
pressure
and debris removal by adjacent cells. It is really only when this
process fails that abnormal growths tend to occur. This may be
via
the production by the original cell of too many apoptosis blocker
proteins,
perhaps as a result of extracellular nutrient or growth factor
availability.
Or via dysregulation of these factors during the original omission of
the
checkpoints prior to daughter cell appearance.
Note that the
complexity of these control paths provide ample
molecular
targets for toxin or toxicant disruptions of these processes. And
while these will most frequently simply cause the damaged cells or
their
daughters to be eliminated by apoptosis and/or auto-immune surveillance
by reticuloendothelial cells such as macrophages, they will
occasionally
result in cellular transformation. If the induction of apoptosis
is widespread or is accompanied by the overt killing of cells via
necrotic
processes (rapid cell death usually due to sudden breach of the cell
membrane
or rapid loss of membrane ion gradients or osmotic gradients followed
by
infiltration with inflamatory cells such as lymphocytes and monocytes)
tissue damage will occur. If the rate at which cell die-off
occurs
exceeds replication of the involved tissue or of fibroblastic (scar)
replacement
tissue, structural and/or functional compromise of the tissue will
result.
Finally, if
compromise of a regulatory tissue such as the cells of
the
thyroid are involved in these cell control modulations, the primary
events
observed will not be the result of the site of the primary
lesion.
Rather, they will involve the lost capacity to adequately communicate
with
the regulatory targets of the thyroid tissues. Heart rhythm may
be
disrupted by depressed thyroxine levels, unexplained weight gain may
occur,
sensitivity to heat and cold will be noted, CNS activity will be
suppressed.
All of these results will demonstrate some level of functional
compromise
and thus be related to a form of morbidity (which may or may not result
in an early death). But none of them will immediately point to
the
actual primary lesion. Only by observing the constellation of
effects
and/or actually measuring blood hormones or the function of the thyroid
gland would this insult be identified. Likewise, loss of muscle
function
or gastric activity may often reflect impacts on nerve tracts rather
than
direct impacts on the muscles or gastric tissues. Toxicological
investigations
must understand and take into account the normal functioning of the
organism
and employ all the tools available when exploring toxic mechanisms and
actual toxic insults.
Assignment Work:
Journal
Citations
- Provide at
least 3 citations gleaned from articles in the toxicology journals
posted.
Calcium
Controls
- Describe the
control circuit for parathyroid hormone, calcitonin, and
cholecalciferol; how
would a blocker of 1[alpha]-hydroxylase impact calcium deposition in
bone?
Project
Ideas
- Generate a
list of 3 possible case studies for possible use in the course project;
associate them with an article, Website, or other document that
justifies
possible interest in them.
© 2005 Kenneth L.
Campbell
 Vascularization
of the anterior pituitary and its association with the structures of
the
human hypothalamus.
Vascularization
of the anterior pituitary and its association with the structures of
the
human hypothalamus.
 Control
of the
fasiculata and reticularis layers of the adrenal cortex by ACTH and of
ACTH by the glucocortical steroids produced by the adrenal cortex
(cortisol
-- human, corticosterone -- rat, mouse). CRH is corticotropin
releasing
hormone, a small protein produced in the hypothalamus. VP is
vasopressin,
a nonapeptide secreted by cells of the posterior pituitary and produced
by the cells of the paraventricular and supraoptic nuclei of the
hypothalamus.
IL-1B is a lymphokine, a protein hormone produced by lymphoid cells.
Control
of the
fasiculata and reticularis layers of the adrenal cortex by ACTH and of
ACTH by the glucocortical steroids produced by the adrenal cortex
(cortisol
-- human, corticosterone -- rat, mouse). CRH is corticotropin
releasing
hormone, a small protein produced in the hypothalamus. VP is
vasopressin,
a nonapeptide secreted by cells of the posterior pituitary and produced
by the cells of the paraventricular and supraoptic nuclei of the
hypothalamus.
IL-1B is a lymphokine, a protein hormone produced by lymphoid cells.
 LH
acts in a simple
negative feedback loop in the male where it acts on the Leydig cells of
the testis to stimulate testosterone production. Testosterone
acts
on peripheral somatic tissues and is a major player in maintaining
adult
sperm production. The steroid also binds to receptors in the
basal
hypothalamus where it suppresses production of the decapeptide hormone
luteinizing hormone releasing hormone/luliberin, LHRH/GnRH. LHRH
in turn stimulates the anterior pituitary gonadotrope cells to produce
LH. In the female, progesterone from the temporary steroidogenic
structure formed from the ovarian follicle following ovulation, the
corpus
luteum, CL, acts in an analogous manner to testosterone. It
stimulates
peripheral tissues such as the uterine lining (endometrium) to
differentiate
in preparation for embryonic implantation but it also acts at the
hypothalamus
to decrease LHRH and subsequently LH production. Since the CL is
dependent on LH action for its function the negative feedback by
progesterone
will normally limit the lifespan of the CL and lead to its functional
and
structural involution. When this happens (in the absence of a
fertilization)
the decline in steroids associated with the CL involution leads to a
rise
in LH (and FSH). These initiate follicular growth in the adult
ovary
and stimulate production of estradiol from the granulosa cells of the
follicle
and the stromal cells of the ovarian tissue. As follicles grow
they
produce more and more estradiol which tends to act in a negative
feedback
manner initially similar to the actions of testosterone and
progesterone
with respect to LH and FSH production. Once a particular
threshold
is reached, however, in the later portion of the preovulatory part of
the
ovarian cycle, estradiol stimulates LH release by increasing the LHRH
receptor
numbers on the gonadotropes and, possibly, by inhibiting its own
negative
feedback action at the level of the hypothalamus. This positive
feedback
results in a spike of LH (and FSH) near the middle of the ovarian cycle
that triggers the shedding of ova from mature follicles and stimulates
conversion of the ovulated follicles into the next crop of CLs.
Glucocortical
steroids and CRH have suppressive actions on LHRH, LH, and sex steroid
productivity.
LH
acts in a simple
negative feedback loop in the male where it acts on the Leydig cells of
the testis to stimulate testosterone production. Testosterone
acts
on peripheral somatic tissues and is a major player in maintaining
adult
sperm production. The steroid also binds to receptors in the
basal
hypothalamus where it suppresses production of the decapeptide hormone
luteinizing hormone releasing hormone/luliberin, LHRH/GnRH. LHRH
in turn stimulates the anterior pituitary gonadotrope cells to produce
LH. In the female, progesterone from the temporary steroidogenic
structure formed from the ovarian follicle following ovulation, the
corpus
luteum, CL, acts in an analogous manner to testosterone. It
stimulates
peripheral tissues such as the uterine lining (endometrium) to
differentiate
in preparation for embryonic implantation but it also acts at the
hypothalamus
to decrease LHRH and subsequently LH production. Since the CL is
dependent on LH action for its function the negative feedback by
progesterone
will normally limit the lifespan of the CL and lead to its functional
and
structural involution. When this happens (in the absence of a
fertilization)
the decline in steroids associated with the CL involution leads to a
rise
in LH (and FSH). These initiate follicular growth in the adult
ovary
and stimulate production of estradiol from the granulosa cells of the
follicle
and the stromal cells of the ovarian tissue. As follicles grow
they
produce more and more estradiol which tends to act in a negative
feedback
manner initially similar to the actions of testosterone and
progesterone
with respect to LH and FSH production. Once a particular
threshold
is reached, however, in the later portion of the preovulatory part of
the
ovarian cycle, estradiol stimulates LH release by increasing the LHRH
receptor
numbers on the gonadotropes and, possibly, by inhibiting its own
negative
feedback action at the level of the hypothalamus. This positive
feedback
results in a spike of LH (and FSH) near the middle of the ovarian cycle
that triggers the shedding of ova from mature follicles and stimulates
conversion of the ovulated follicles into the next crop of CLs.
Glucocortical
steroids and CRH have suppressive actions on LHRH, LH, and sex steroid
productivity.
 The
FSH control
cycle is intimately tied to that of LH and is only slightly less
complex.
In the male testosterone suppresses LHRH, LH, and FSH production while
being directly influenced only by LH. FSH stimulates the Sertoli
cells of the seminiferous tubules to produce along with the proteins
and
metabolic products needed to help support spermatogenesis, a protein
hormone,
inhibin, that acts in a direct feedback manner on the
gonadotropes.
Primary control of FSH is due to steroid feedback with only about 20%
of
levels critically dependent on inhibin. In the female, granulosa
cells produce inhibin in response to FSH as well as estradiol. So
growing follices are actually producing both a protein and a steroid
that
help suppress FSH production even as they depend on FSH actions to
continue
their growth and development. Again, primary control of FSH is
due
to steroid, rather than inhibin feedback. Once the estradiol
threshold
necessary to convert estradiol negative feedback into positive feedback
is reached, FSH levels also rise to a mid-cycle peak and then decline
as
progesterone takes over to suppress LHRH, LH, and FSH levels.
The
FSH control
cycle is intimately tied to that of LH and is only slightly less
complex.
In the male testosterone suppresses LHRH, LH, and FSH production while
being directly influenced only by LH. FSH stimulates the Sertoli
cells of the seminiferous tubules to produce along with the proteins
and
metabolic products needed to help support spermatogenesis, a protein
hormone,
inhibin, that acts in a direct feedback manner on the
gonadotropes.
Primary control of FSH is due to steroid feedback with only about 20%
of
levels critically dependent on inhibin. In the female, granulosa
cells produce inhibin in response to FSH as well as estradiol. So
growing follices are actually producing both a protein and a steroid
that
help suppress FSH production even as they depend on FSH actions to
continue
their growth and development. Again, primary control of FSH is
due
to steroid, rather than inhibin feedback. Once the estradiol
threshold
necessary to convert estradiol negative feedback into positive feedback
is reached, FSH levels also rise to a mid-cycle peak and then decline
as
progesterone takes over to suppress LHRH, LH, and FSH levels.
 Control
of
thyroid function involves inhibition of the thyroptropes of the
anterior
pituitary by circulating thyroxine which acts directly as it does to
promote
growth and maintain metabolism on many somatic tissues in the
periphery.
Additionally, thyroxine can inhibit hypothalamically produced
TRH.
Somatostatin, a tetradecapeptide generated in the hypothalamus, can act
as a secondary controller by limiting the stimulatory actions of
TRH.
TSH from the thyrotrope then acts selectively on the follicular
epithelial
cells of the thyroid to stimulate the synthesis and release of
thryonines,
and especially thyroxine.
Control
of
thyroid function involves inhibition of the thyroptropes of the
anterior
pituitary by circulating thyroxine which acts directly as it does to
promote
growth and maintain metabolism on many somatic tissues in the
periphery.
Additionally, thyroxine can inhibit hypothalamically produced
TRH.
Somatostatin, a tetradecapeptide generated in the hypothalamus, can act
as a secondary controller by limiting the stimulatory actions of
TRH.
TSH from the thyrotrope then acts selectively on the follicular
epithelial
cells of the thyroid to stimulate the synthesis and release of
thryonines,
and especially thyroxine.